Why Is Genotypic Precision Medicine Challenging in Advanced Prostate Cancer?
Ulka Vaishampayan, MBBS, FAB
Dr. Vaishampayan examines the challenges associated with traditional genotypic precision medicine that underscore the need for novel approaches to increase and simplify the use of precision medicine in advanced prostate cancer.
Precision medicine has traditionally relied on genetic biomarkers to enable clinical decision making.1,2 However, the use of genotypic biomarkers in advanced prostate cancer is challenging because of the complexity and heterogeneity of the disease.3-7
Heterogeneity in advanced prostate cancer presents at several levels:
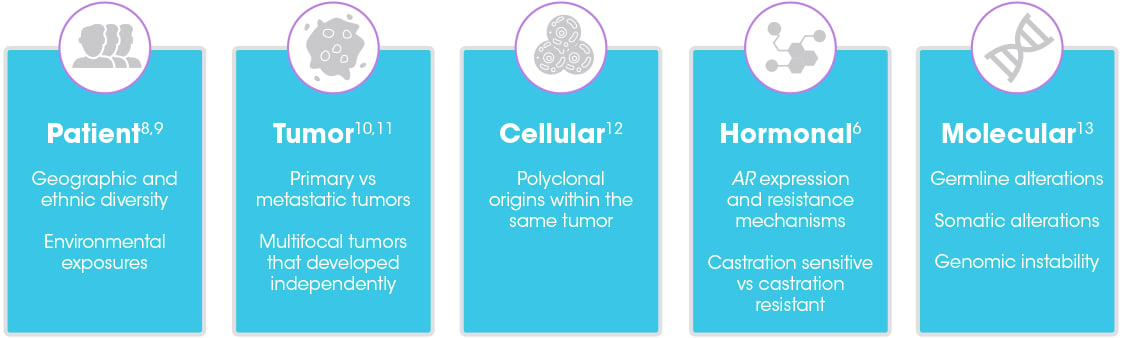
The heterogeneity of advanced prostate cancer has been attributed to 2 components3-6,13:
- Genomic instability of advancing disease
- Treatment-induced selective pressures, which can lead to genetic mutations and resistance
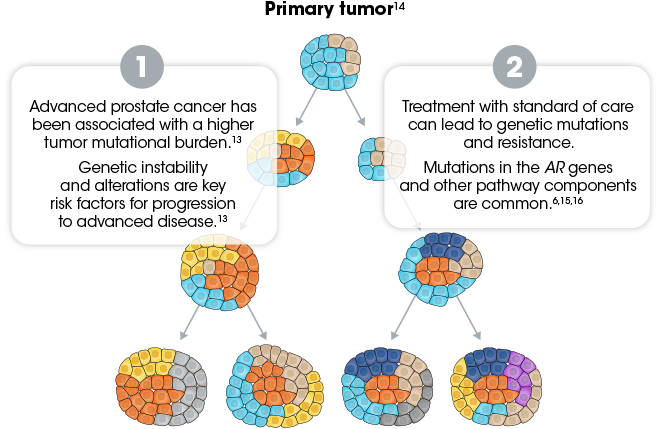
AR, androgen receptor.
Due to the heterogeneity of prostate cancer, few widespread driver mutations have been identified, further complicating the use of genotypic precision medicine.17,18
Taken together, the heterogeneity of advanced disease and lack of widespread driver mutations underlie the need for novel precision medicine approaches in prostate cancer, such as the use of phenotypic biomarkers.19,20
Learn more:


